Notation
Cations
Anions
Formula subscripts
1
2
2
3
9
Elements
Tl3+
Cs1+
Ba2+
Cs1+
Ca2+
Tl3+
Cu2+
O2−
F1-
Substitution
0.908
0.092
2
−
1.862
0.138
3
8.82
−
Charge
+2.724
0.092
+4
−
+3.724
+0.414
+6
−17.64
−
h+ = 0.686

Tco = 136 K
Substitution
0.75
0.25
2
−
1.862
0.138
3
8.33
0.67
Charge
+2.25
+0.25
+4
−
+3.724
+0.414
+6
−16.6
−0.7
h+ = 0.692
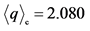
Tco = 146 K
Substitution
0.5
0.5
2
−
1.862
0.138
3
7.82
1.18
Charge
+1.5
+0.5
+4
−
+3.724
+0.414
+6
−15.64
−1.18
h+ = 0.682
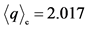
Tco = 166 K
Substitution
−
1
0.9
1.1
2
−
3
5.59
3.41
Charge
−
+1
+1.8
+1.1
+4
−
+6
−11.18
−3.41
h+ = 0.690

Tco = 300 K