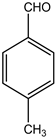
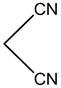
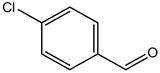
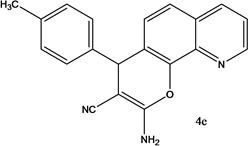S.No
Reactants
Pyranoquinoline derivatives
1
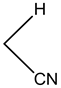
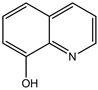
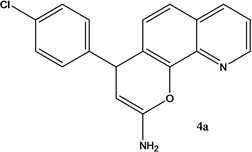
4-(4-chlorohenyl)-4H-pyrano[3,2-h]quinoline-2-amine (4a)
White solid, yield; 92%, IR (KBr, υmax cm−1); 3424 (NH2 str), 3049 (-CH str), 1592 (-C=N str), 1111 (-C-O-C- str); 1H NMR (CDCl3-400 MHz, δ ppm); 8.7 - 8.8 (d, Ar-H), 8.0 - 8.1 (d, Ar-H), 8.2 (d, Ar-H), 7.4 - 7.5 (m, Ar-H), 7.3 (d, Ar-H), 7.2 - 7.3 (s, NH2), 7.1 - 7.2 (d, Ar-H), 5.2 - 5.3 (d, CH-pyran ring), 4.2 - 4.3 (d, ethylene proton); ESMS: 309 [M + 1].
2

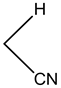
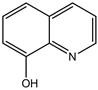
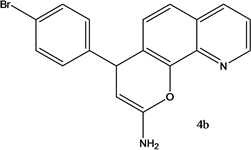
4-(4-Bromohenyl)-4H-pyrano[3,2-h]quinoline-2-amine (4b)
White solid, yield; 88%, IR (KBr, υmax cm−1) ; 3627 (NH2 str), 3091 (-CH str), 1584 (-C=N str), 1223 (-C-O-C-str); 1H NMR (CDCl3-400 MHz, δ ppm); 8.9 (d, Ar-H), 8.0 (d, Ar-H), 8.1 (d, Ar-H), 7.3 (m, Ar-H), 7.3 - 7.4 (d, Ar-H), 7.1 (s, NH2), 7.2 - 7.3 (d, Ar-H),7.3 (d, Ar-H), 7.2 (d, Ar-H), 7.1 (d, Ar-H) 5.0 (d, CH-pyran ring), 4.1 - 4.2 (d, ethylene proton); ESMS: 353 [M + 1].
3

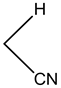
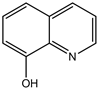
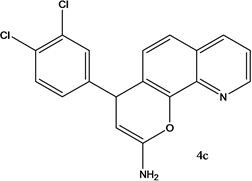
4-(3,4-dichlorohenyl)-4H-pyrano[3,2-h]quinoline-2-amine (4c)
White solid, yield; 86%, IR (KBr, υmax cm−1) ; 3421 (NH2 str), 3089 (-CH str), 1588 (-C=N str), 1280 (-C-O-C- str); 1H NMR (CDCl3-400 MHz, δ ppm); 7.9 - 8.0 (d, Ar-H), 8.1 - 8.2 (d, Ar-H), 7.4 (d, Ar-H), 7.5 - 7.6 (m, Ar-H), 7.6 - 7.7 (d, Ar-H), 7.5 (m, Ar-H), 7.3 (s, NH2), 5.2 - 5.3 (d, CH-pyran ring), 4.1 - 4.3 (d, ethylene proton); ESMS: 344 [M + 1].
4
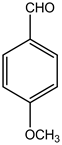
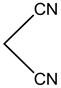
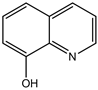
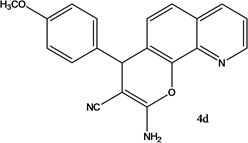
2-amino-4-(4-methoxyphenyl)-4H-pyrano[3,2-h]quinoline-3-carbonitrile (4d)
White solid, yield; 92%, IR (KBr, υmax cm−1); 3421 (NH2 str), 3027 (-CH str), 2221 (-CN), 1604 (-C=N str), 1236 (-C-O-C- str); 1H NMR (CDCl3-400 MHz, δ ppm); 7.9 (d, Ar-H), 7.6 - 7.7 (d, Ar-H), 7.8 (d, Ar-H), 7.5 (m, Ar-H), 7.6 (d, Ar-H),7.3 (s, NH2), 7.0 (d, Ar-H), 6.8 - 6.9 (d, Ar-H), 4.7 - 4.8 (s, CH-pyran ring), 3.9 (s, 3H, OCH3); ESMS: 330 [M + 1].
5