Chloride
Melting point K
Standard free energy of formation ∆G˚298 KJ∙mol−1 of 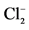
Vapor pressure at 298 K KPa
FeCl2(s)
950
−378.05
≈10−9 [a]
SnCl2(s)
520
−363.77
<10−5
SnCl4(l)
240
−294.20
3.42
| Chloride | Melting point K | Standard free energy of formation ∆G˚298 KJ∙mol−1 of | Vapor pressure at 298 K KPa |
| FeCl2(s) | 950 | −378.05 | ≈10−9 [a] |
| SnCl2(s) | 520 | −363.77 | <10−5 |
| SnCl4(l) | 240 | −294.20 | 3.42 |