Adsorbents (I)
Absorbents (II)
Reactants (III)
Kinetics
Kinetics
Kinetics
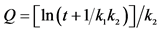 , where k1 and k2 are constants [28] [29] .
, where k1 and k2 are constants [28] [29] .
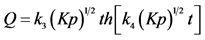 , if the process is limited by the dissociation of molecules
, if the process is limited by the dissociation of molecules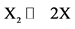 ;
;
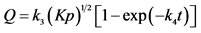 , if the process is limited by the transition
, if the process is limited by the transition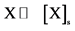 ;
;
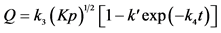 , if the process is limited by the diffusion of atoms of X in [X]s.
, if the process is limited by the diffusion of atoms of X in [X]s.
Here K is an equilibrium constant, p is vapor pressure of gas X2 above the getter, k3, k4 and k′ are coefficients, [X]s is solid solution of gas X in metal [18] .
Q = k5(t1/2), or Q = k6t depending on the nature of the metal. Here k5 and k6 are rate constants [16] - [18]